The antiquated Greek thinker Aristotle is credited with saying that nature loathes a vacuum.
For Thomas Strawmier, the vacuum was the vacant field he continued driving past on the grounds of the year-old Creighton University Medical Center-University Campus at 24th and Cuming Streets.
“I figured, ‘We should put something there,’ ” said Strawmier, a medical caretaker specialist at the facility.
Realizing that the facility serves an assorted gathering of patients, some of whom experience difficulty getting new veggies, he proposed a network plant.
It could give some deliver to patients — even offer beds for neighbors who need to become their own — and fill in as a showing site for nutritionists, physical advisors and social wellbeing experts. There they could convey exercises on adhering to good diet, damage free planting and stress administration.
So half a month prior, Strawmier and a bunch of facility associates — and their children — filled and planted five raised garden beds, introduced by accomplice City Sprouts, on the site.
“Everyone cherished the thought immediately,” said Nicki Blodgett, a therapeutic aide at the facility who brought child Ryder, 6, and little girl, Lexi, 7, to encourage plant. “We do as such numerous things here to connect the network with assets. This was simply one more advance in that.”
Neighborhood wellbeing frameworks long have upheld and joined forces with network cultivate gatherings. Be that as it may, the garden has all the earmarks of being the first specifically settled by a wellbeing framework.
Such endeavors are a piece of a developing spotlight on keeping individuals well, a concentration that goes past advising individuals to eat solid eating methodologies — now medicinal services suppliers are demonstrating to them generally accepted methods to do it through cooking classes and so forth. In a few sections of the nation, a couple of human services designs even have started giving medicinally custom-made suppers through “nourishment as drug” programs.
Audrey Matthews, more advantageous networks facilitator for CHI Health, said the association needs the University Campus garden to develop. While the first beds will be planted by staff, the wellbeing framework would like to include more for neighbors and turn into an undeniable network plant where inhabitants and staff can work and get to know each other.
“The more open doors we can accommodate the network to go to the property and be locked in, the better,” she said.

Independently, Matthews stated, CHI Health likewise is working with City Sprouts, the Latino Center of the Midlands and OneWorld Community Health Centers to help enhance access to new create in South Omaha.
In light of a model created in a Denver suburb, the South Omaha program calls for procuring a network wellbeing laborer to distinguish and work with families confronting nourishment weakness, implying that they may not generally know when or where they’ll eat their next dinner.
The laborer, who will make home visits, will instruct families about urban farming and help them plant cultivates in their own particular lawns or at City Sprouts South, the network cultivating gathering’s greenery enclosure close twentieth and N Streets.
The $326,000 venture is financed by a two-year allow from CHI Health’s parent organization, and also extra gifts and in-kind commitments.
Establishment of the University Campus plant cost about $2,000, with stores originating from CHI Health’s people group advantage office.
Matthews said coordinators intend to start distinguishing families in South Omaha this fall. While the underlying concentration is sustenance get to, authorities with the Denver-region program have discovered that the connections the wellbeing laborers work with families inevitably enable them to recognize and address other social needs — say, worry over having the capacity to pay lease — that can influence wellbeing.
Generally, she stated, medicinal services has been directed inside healing facilities and centers. In any case, inquire about demonstrates that human services suppliers need to go past those dividers.
“We’re eager to have the capacity to go and meet the families where they’re at and give those administrations,” Matthews said.
Albert Varas, official executive of the Latino Center of the Midlands, said network cultivating has turned out to be prevalent in north Omaha yet at the same time is genuinely restricted in South Omaha.
An evaluation of the middle’s customers demonstrated a requirement for sustenance instruction and stress administration, he said. Cultivating can address both, notwithstanding helping individuals save money on basic supply bills.
The Latino Center, as a major aspect of a different exertion, as of now has an exhibition garden to demonstrate inhabitants how it’s done — raised beds set up by City Sprouts and supported by CHI Health. “Our beds are ablaze,” Varas said. “They’re stacked.”
Under CHI Health’s South Omaha venture, a property holder has consented to plant a home show cultivate so would-be plant specialists can perceive how a patio plot functions.
Roxanne Draper, City Sprouts official executive, said the local gathering will give training to the individuals who need it. Its cultivating classes are offered in Spanish.
“We’re anticipating a great deal of development in South Omaha,” she said.
Strawmier and his partners at the University Campus, interim, have a lot of thoughts of their own for their garden.
Suppliers there observe loads of patients with ceaseless diseases who could profit by cultivating, he said. Having neighbors develop vegetables from their nations of origin would include some social enthusiasm too. Inevitably, he’d jump at the chance to include some organic product trees.
“A great deal of conceivable outcomes,” he stated, as he completed the process of scooping soil into the new beds. “Yet, this is the place we begin.”
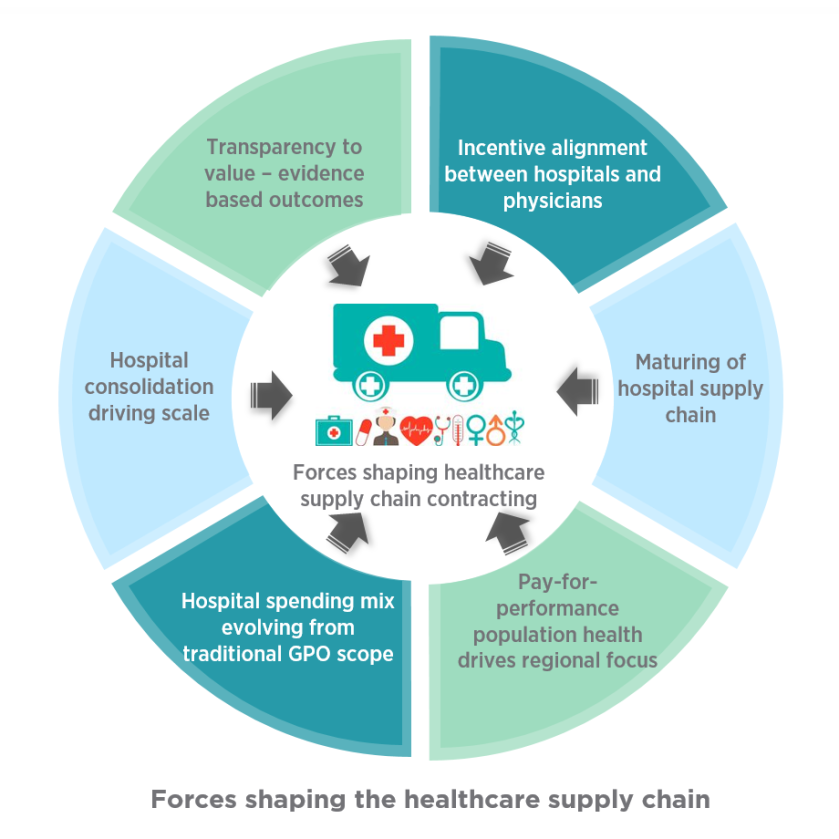
![Healthcare Supply Chain Reaches Master [Level Ranking]](https://compliance4all14.files.wordpress.com/2018/11/12.jpg?w=852)